Understanding Blood Buffer Systems in Animals
Introduction to Buffer Systems
Buffer systems are chemical solutions that resist changes in pH when acids or bases are added. They play a critical role in biological systems. The human body, like many animals, needs to keep its blood pH between 7.35 and 7.45. Deviations from this range can lead to severe health issues.
Importance of pH Balance
The pH scale ranges from 0 to 14, with 7 being neutral. A pH lower than 7 indicates acidity, while a pH above 7 indicates alkalinity. In animals, enzymes and biochemical reactions depend on a stable pH. Therefore, maintaining this balance is vital for survival.
Major Blood Buffer Systems
Carbonic Acid-Bicarbonate Buffer System
The carbonic acid-bicarbonate buffer system is the most significant buffering system in animal blood. It helps maintain pH homeostasis through the following reaction:
\ce{CO2+H2O<=>H2CO3<=>H++HCO3^-}
In this system:
- Carbon dioxide (CO2) combines with water (H2O) to form carbonic acid (H2CO3).
- Carbonic acid can dissociate into hydrogen ions (H+) and bicarbonate ions (HCO3^-).
This dynamic equilibrium allows the body to respond quickly to changes in pH. For example, if excess hydrogen ions are present (making the blood more acidic), bicarbonate can neutralize them by forming carbonic acid.
Protein Buffers
Proteins also serve as buffers in the blood. Hemoglobin is a prime example. It binds to hydrogen ions and carbon dioxide during gas exchange. This process helps regulate blood pH effectively.
- Hemoglobin’s Role: When hemoglobin releases oxygen to tissues, it can pick up hydrogen ions. This reaction helps maintain a stable pH during metabolic activities.
Other proteins in blood plasma, such as albumin, also contribute to buffering capacity. Collectively, proteins account for about two-thirds of the buffering power in blood.
Phosphate Buffer System
The phosphate buffer system is another important mechanism for regulating blood pH. It involves two forms of phosphate:
- Dihydrogen phosphate (\ce{H2PO4^-})
- Hydrogen phosphate (\ce{HPO4^{2-}})
These phosphates can react with excess acids or bases to stabilize pH levels. While not as dominant as the bicarbonate system, the phosphate buffer plays a role in cellular environments and contributes to overall acid-base balance.
Mechanisms of Regulation
Respiratory Control
The respiratory system helps regulate blood pH by controlling carbon dioxide levels. When CO2 levels rise due to increased metabolic activity:
- The body increases breathing rate.
- More CO2 is expelled.
- This process reduces carbonic acid levels and raises blood pH.
Conversely, if CO2 levels drop (e.g., during hyperventilation), blood becomes more acidic as carbonic acid increases.
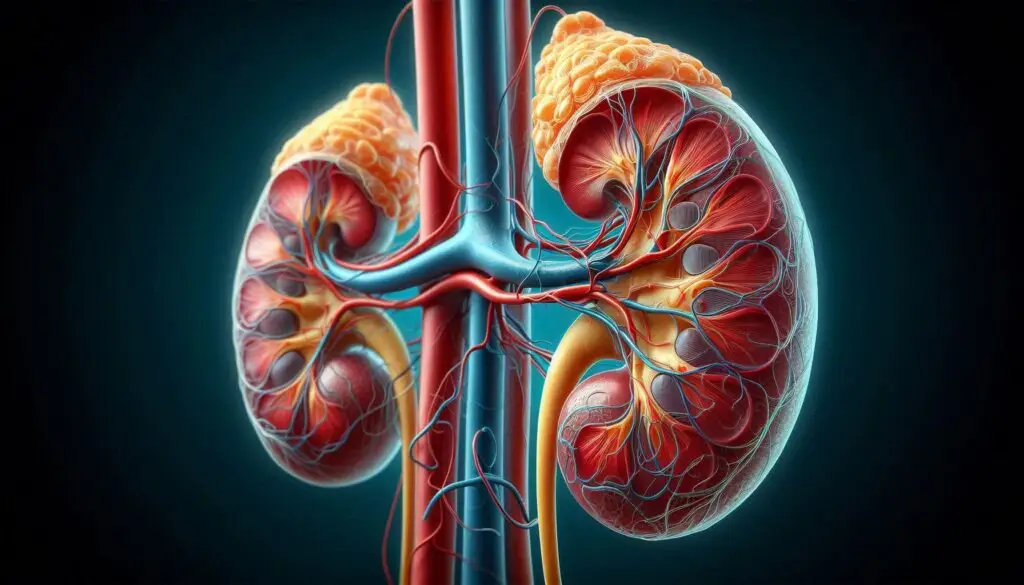
Renal Control
The kidneys play a long-term role in maintaining acid-base balance by:
- Excreting hydrogen ions.
- Reabsorbing bicarbonate from urine.
This process takes longer than respiratory adjustments but is essential for maintaining stable pH over time.
Factors Affecting Buffer Systems
Several factors can influence the effectiveness of buffer systems:
- Metabolic Activity: Increased physical activity generates more CO2 and lactic acid, challenging buffer systems.
- Diet: High-protein diets can produce more acids, affecting overall pH balance.
- Health Conditions: Conditions like diabetes or kidney disease can disrupt normal buffering mechanisms.
Conclusion
Buffer systems are vital for maintaining proper blood pH in animals. The carbonic acid-bicarbonate system stands out as the primary regulator, supported by protein and phosphate buffers. Understanding these mechanisms highlights their importance not only for individual health but also for overall physiological function.
For more pearls of Vets Wisdom:
https://wiseias.com/partitioning-of-food-energy-within-animals/





Responses